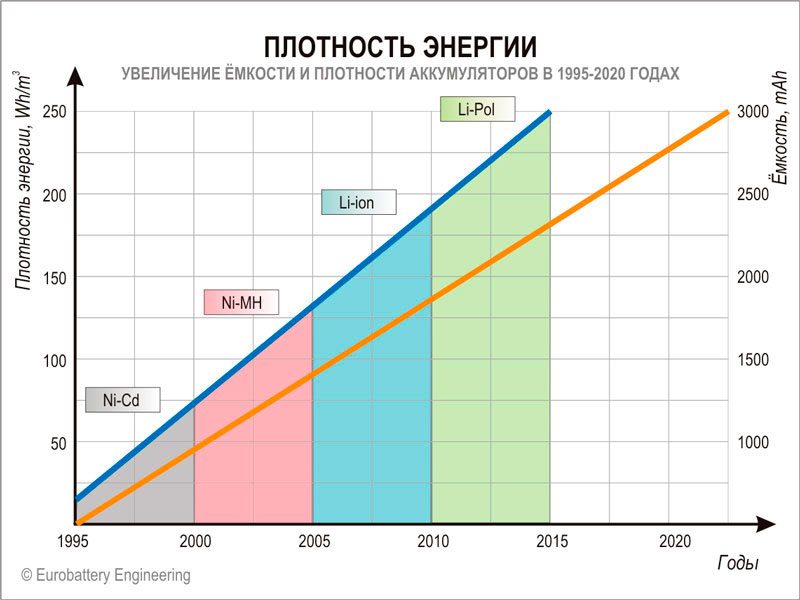
Stanford: Re fokolitse boima ba babokelli ba hona joale liseleng tsa lithium-ion ka liperesente tse 80. Matla a matla a eketseha ka liperesente tse 16-26.
Bo-rasaense ba Univesithing ea Stanford le Setsi sa Stanford Linear Accelerator (SLAC) ba ile ba etsa qeto ea ho fokotsa lisele tsa lithium-ion ho fokotsa boima ba tsona 'me kahoo ba eketsa matla a polokelo. E le hore ba etse sena, ba ile ba tsosolosa lihlopha tsa thepa ka ntle: ho e-na le maqephe a sephara a koporo kapa aluminium, ba ne ba sebelisa likhoele tse moqotetsane tsa tšepe, tse tlatsitsoeng ka lera la polymer.
Matla a phahameng a matla ho Li-ion ntle le litšenyehelo tse phahameng tsa matsete
Sele e 'ngoe le e' ngoe ea Li-ion ke moqolo o nang le sekhahla sa ho ntša / ho ntša, elektrode, electrolyte, electrode, le mokelli oa hona joale ka tatellano eo. Likarolo tse ka ntle ke foil ea tšepe e entsoeng ka koporo kapa aluminium. Li lumella lielektrone ho tsoa ka seleng ebe li khutlela ho eona.
Bo-rasaense ba tsoang Stanford le SLAC ba ile ba etsa qeto ea ho tsepamisa maikutlo ho babokelli, hobane boima ba bona hangata ke karolo ea mashome a 'maloa lekholong ea boima ba khokahanyo eohle. Sebakeng sa maqephe a koporo, ba ne ba sebelisa lifilimi tsa polymer tse nang le likhoele tse moqotetsane tsa koporo. Ho ile ha fumaneha hore ho ne ho ka khoneha ho fokotsa boima ba babokelli ka liperesente tse 80:
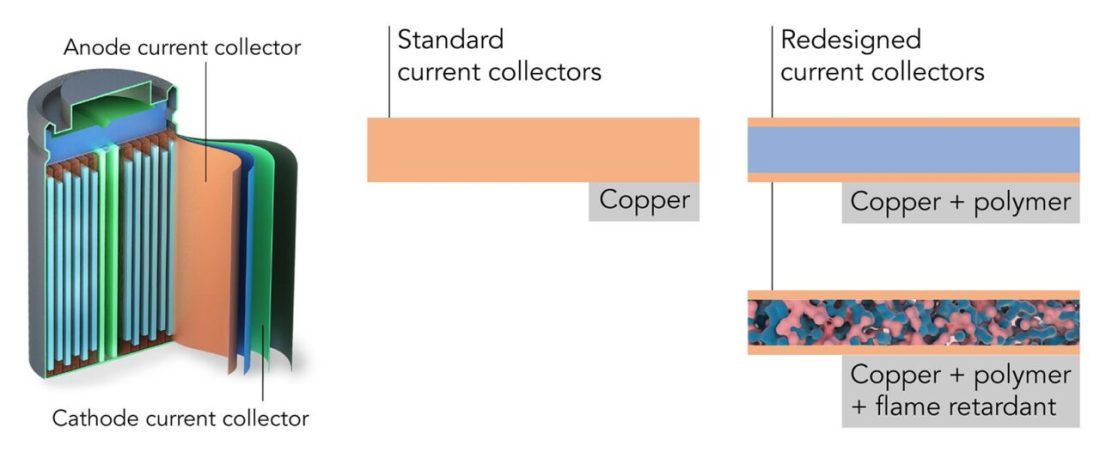
The classic cylindrical lithium-ion cell ke moqolo o molelele o nang le likarolo tse 'maloa. Bo-ramahlale ba tsoang Stanford le SLAC ba fokolitse likarolo tse bokellang litefiso le ho li tsamaisa - babokelli ba hajoale. Ho e-na le maqephe a koporo, ba ne ba sebelisa maqephe a polymer-koporo a nang le lik'hemik'hale tse sa cheng (c) Yusheng Ye / Stanford University
Ha se eona feela: metsoako ea lik'hemik'hale e thibelang ho chesa e ka eketsoa ho polymer, ebe ho chesa ha lintho tse ka tlaase ho tsamaea le boima bo tlaase:

Ho chesa ha foil ea koporo e sebelisoang ka seleng ea khale ea lithium-ion le mokelli e entsoeng ke bafuputsi ba Amerika (c) Yusheng Ye / Stanford University
Bafuputsi ba re babokelli ba nchafalitsoeng ba ka eketsa matla a matla a khoheli a lisele ka karolo ea 16-26 lekholong (= 16-26 lekholong ea matla a eketsehileng bakeng sa boima bo tšoanang ba yuniti). Ho bolela seo betri ea molumo o tšoanang le matla a matla a ka ba karolo ea 20 lekholong ho feta ea hona joale.
Ho bile le liteko tsa ho ntlafatsa letamo nakong e fetileng, empa ho li fetola ho lebisitse litlamorao tse neng li sa lebelloa. Lisele li ile tsa fetoha tse sa tsitsang kapa ho ne ho hlokahala electrolyte [e theko e boima haholo]. Phapang e entsoeng ke bo-ramahlale ba Stanford ha e bonahale e baka mathata a joalo.
Lintlafatso tsena li liphuputsong tsa pele, kahoo u se ke oa lebella hore li tla fihla 'marakeng pele ho 2023. Leha ho le joalo, li shebahala li tšepisa.
Ke habohlokoa ho eketsa hore Tesla o boetse o na le maikutlo a thahasellisang a ho bokella tefiso ea likarolo tsa tšepe. Ho e-na le ho sebelisa likhoele tse tšesaane tsa koporo ka bolelele bohle ba moqolo ebe o li isa sebakeng se le seng (bohareng), o li ntša hang-hang a sebelisa moeli o sehiloeng. Sena se etsa hore litefiso li tsamaee sebaka se senyenyane haholo (ho hanyetsa!), 'me koporo e fana ka phetisetso e eketsehileng ea mocheso ho ea ka ntle:
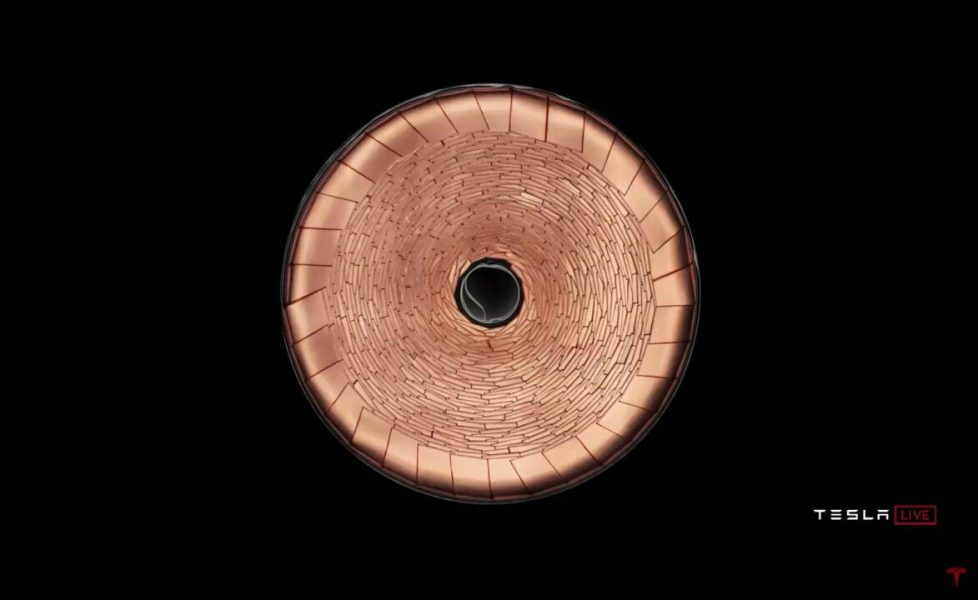
> Na lisele tse 4680 tsa libeteri tse ncha tsa Tesla li tla folisoa ho tloha holimo le tlase? Ho tsoa tlase feela?
Sena se ka u khahla:
